What is the structure of the Atom?
oooo
oooo

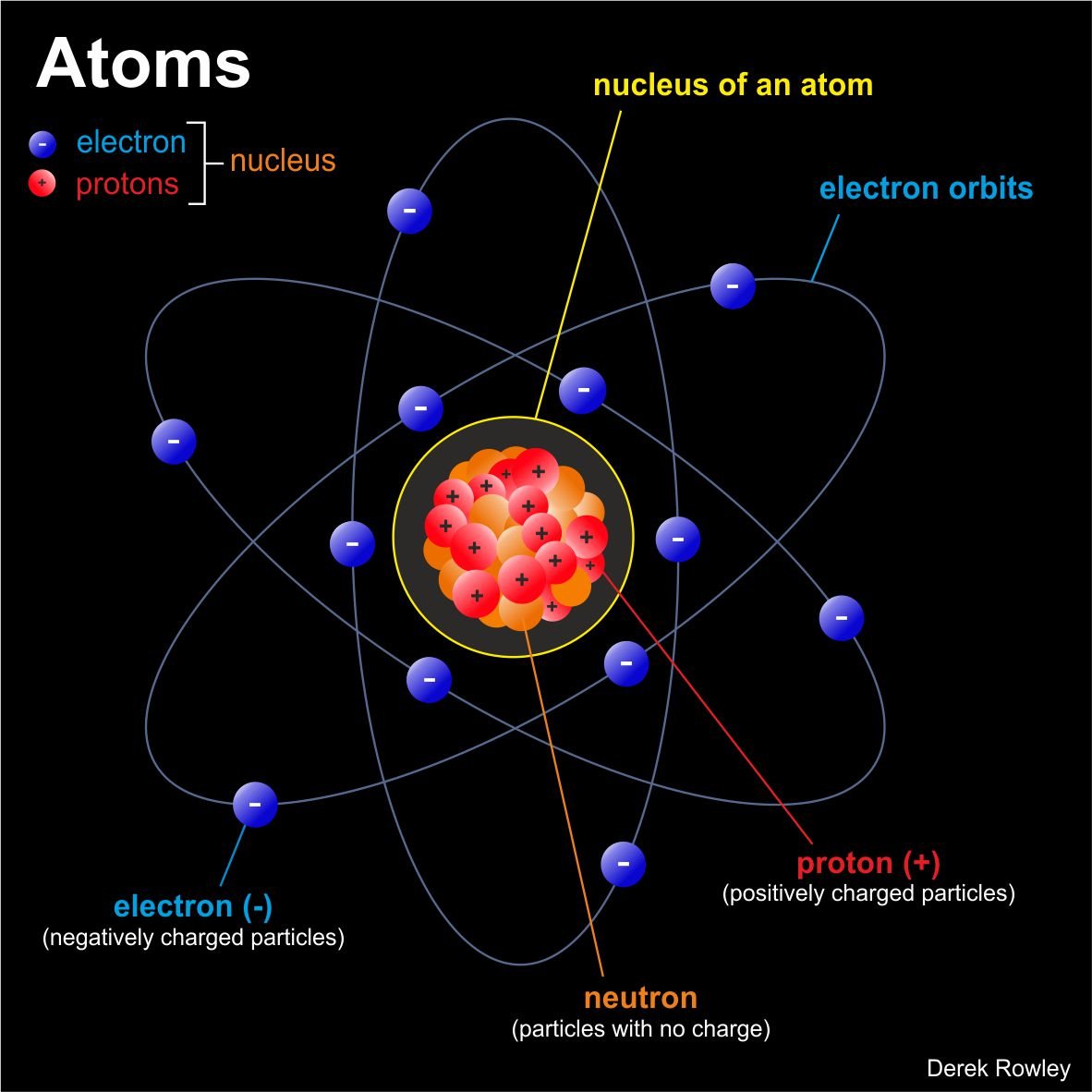
Before you start – Parts of an Atom Remember PEN!
oooo
- Proton
A tiny particle that exists in the nucleus of every atom and has a positive electrical charge.
o - Neutron
A tiny particle of matter that has no electrical charge and is part of the nucleus of all atoms except hydrogen atoms.
o - Electron
A tiny particle that has a negative electrical charge and travels around the nucleus of an atom.
o

BSL Version
oooo
oooo
oooo
What is atomic structure?
- Simply put, atomic structure refers to the structure of the atom.
o - “Remember PEN” – an atom comprises three different particles, known as protons, electrons and neutrons.
o


- The protons and neutrons come together in the centre of the atom to form the nucleus.
o - As protons are positively charged and neutrons have no electrical charge, the overall charge of a nucleus is positive.
o - Electrons circle the nucleus, and have a negative charge.

BSL Version
oooo

What are protons?
- Protons are positively charged particles found inside the nucleus of an atom.
o
o - They help to identify the atom – different elements will have a different number of protons in the nucleus.
o
o - The number of protons in an atom is signified by the atomic number on the periodic table.
o

BSL Version
oooo

What are neutrons?
- Neutrons are also found in the nucleus of the atom.
o
o - They have no electrical charge and contribute to the atomic mass.
o - the core of an atom is the nucleus – made two kinds of sub-atomic particle; protons and neutrons.
o

BSL Version
oooo

oooo
What is electrons?
- Swooshing around the nucleus are even tinier particles called electrons.
o
o - Electrons are negatively charged particles that circle the nucleus of the atom.
o
o - They circle so fast that they create what’s called an electron cloud around the nucleus.
o
o - Their mass is so small, it is deemed insignificant.
o
o - As such, they do not contribute to the mass of an atom.

BSL Version
oooo

What is atomic structure?
- Simply put, atomic structure refers to the structure of the atom.
o - An atom comprises three different particles, known as protons, neutrons, and electrons.
o - The protons and neutrons come together in the centre of the atom to form the nucleus.
o - As protons are positively charged and neutrons have no charge, the overall charge of a nucleus is positive.
o - Electrons circle the nucleus, and have a negative charge.
o

BSL Version
oooo
oooo
Sub-atomic Particle
o
In the centre of the atom is the nucleus,
made up of equal numbers of protons and neutrons.
oooo
oooo
- matter is made from small particles called atoms – they are the building blocks of the Universe.
o - atoms are so extremely small that one million could fit on the full stop at the end of this sentence.
o - atoms are the smallest identifiable piece of a chemical element.
o - many different atoms as there are elements – The Periodic Table of the Elements.
o - atoms are mostly empty space with tiny subatomic particles (subatomic means “smaller than an atom”).
o
o - swooshing around the nucleus are even tinier particles called electrons.
o - electrons have a negative electrical charge; protons have a positive charge – electrons are held to the nucleus by electrical attraction.
o - under certain conditions – one atom can be split into more than 200 kinds of short-lived sub-atomic particle of nucleus are made from various tiny particles called quarks.

BSL Version
oooo
oooo
oooo
oooo
oooo
Back to The Universe / next to Nuclear Energy page.