oooo
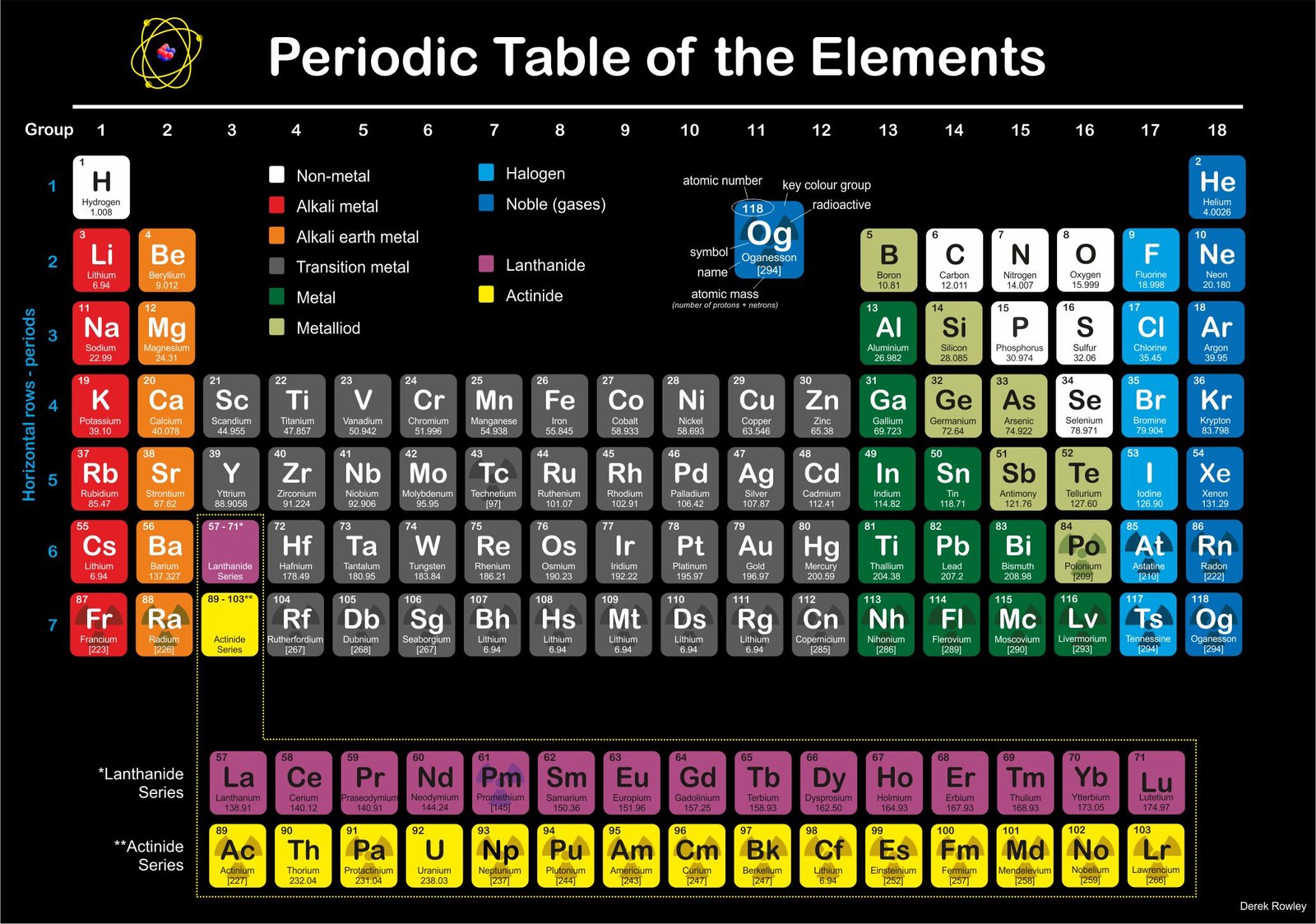
The Periodic Table of chemical elements represents
the material – “ingredients lists” of the present-day Universe.
o
oooo
oooo
oooo
oooo
oooo
Periodic Table
oooo
oooo
- elements are arranged according to their atomic number;
– the same number of protons in the nucleus.
o
o - in horizontal row – it’s called “periods and contain elements” that the same number of electron shells around the nucleus.
o
o - electron circle the nucleus at different distances depending on how much energy they have.

BSL Version
oooo
oooo
Vertical Columns ~ Group 1 to 18
oooo

oooo
oooo
oooo
oooo
oooo
oooo
oooo
oooo
Elements Organised by Group 1 to 18
o
- Group 1 – Hydrogen / alkali metals (Group A)
- Group 2 – alkali earth metals (Group B)
- Group 3 – transition metals (Group C)
- Group 4 – transition metals (Group C)
- Group 5 – transition metals (Group C)
- Group 6 – transition metals (Group C)
- Group 7 – transition metals (Group C)
- Group 8 – transition metals (Group C)
- Group 9 – transition metals (Group C)
- Group 10 – transition metals (Group C)
- Group 11 – transition metals (Group C)
- Group 12 – transition metals (Group C)
- Group 13 – boron (Group D – Icosagen)
- Group 14 – carbon (Group E – Crystallogens)
- Group 15 – nitrogen (Group F – Pnictogen)
- Group 16 – oxygen (Group G – Chalcogen)
- Group 17 – halogens (Group H)
- Group 18 – noble gases (Group I)
o
oooo

BSL Version
oooo
(Group A to Group I ~ Source by Wikipedia)
oooo
oooo
oooo
oooo
Understanding about the Periodic table
o
- elements are arranged according to their atomic number, which is the same as the number of protons in the nucleus.
o
o - the horizonal rows are called periods ~ contain elements that have the same number of electron shells around the nucleus.
o
o - there are nine vertical columns that called groups ~ these contain elements that have similar chemical properties; such as group I on the far right that contains the inert noble gases.
o
o - Group 1
– contains the alkali metals; surprisingly ~ hydrogen is chemically an alkali metal.
o - Group 2
– contains the alkali earth metals.
o - Group 2 to Group 16 – contains poor metals and non-metals; with the halogens in group 17.
o - Group 2 to Group 12 – metallic transition elements (which have incomplete inner electron shells) – located in the middle between group 2 and group 13.
oooo
oooo
oooo
oooo

BSL Version
oooo
oooo
Back to The Universe / next to Periodic Table page.