oooo
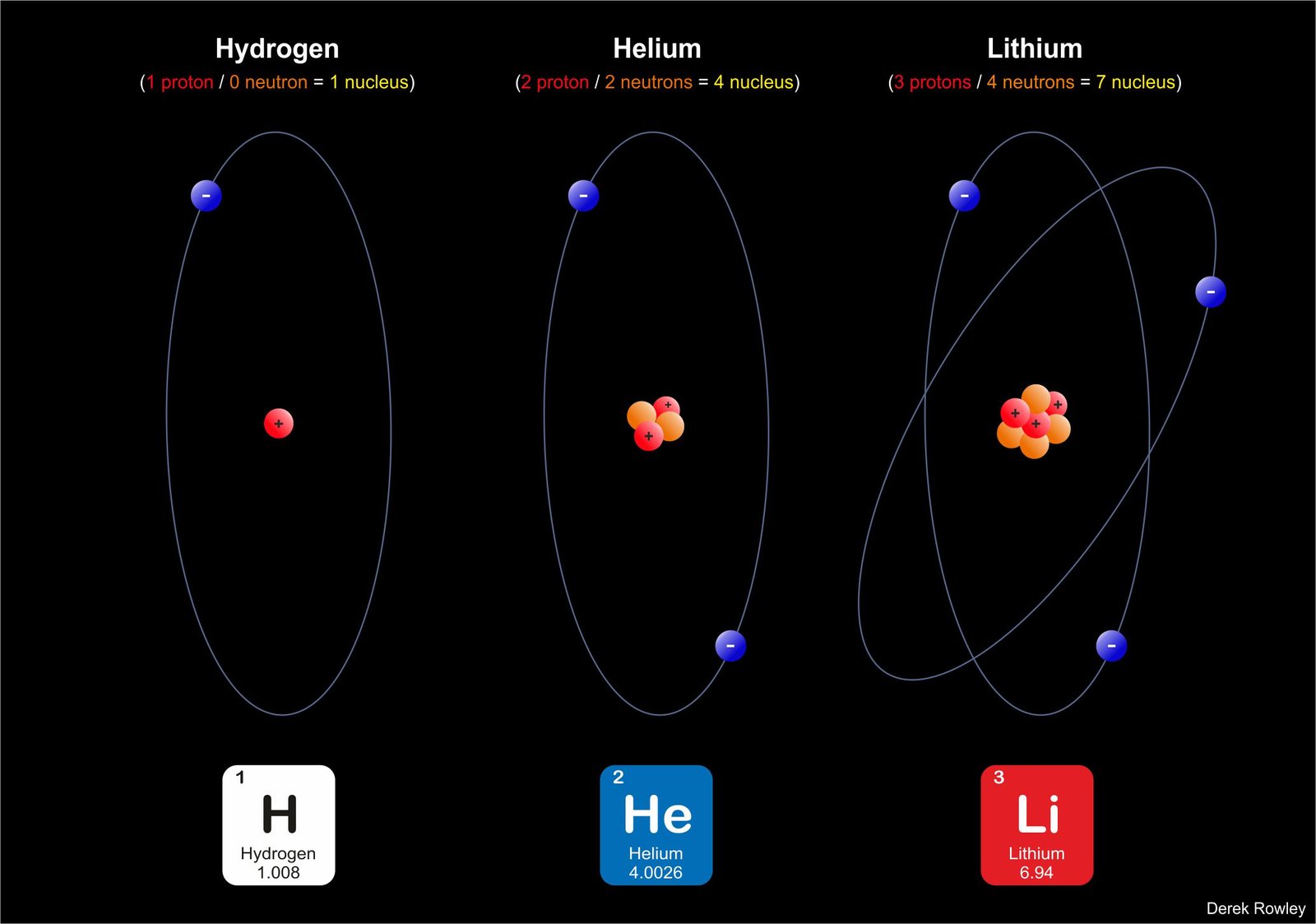
- All the atoms that make up a single element have the same number of protons.
o - All atoms except those of the simplest form of hydrogen also contain neutrons.
o - Electrons circle the nucleus at different distances depending on how much energy they have.

BSL Version
oooo
oooo
Building Elements
oooo
oooo
oooo
oooo
oooo
What is Building Elements?
o
- The basic chemicals of the Universe are elements – material produced by or used in a reaction involving changes in atoms, and they cannot be broken down into other substances – solid mass.
o
o - elements are formed entirely of atoms that contain the same number of protons in their nucleus for example;
– for example:
………– all hydrogen atoms have one proton.
………– all helium atoms have two proton.
………– all lithium atoms have three proton.
o
o - more than 100 elements are known.
o
o - simplest and lightest elements;
– hydrogen and helium
– formed nearly 14 billion years ago in the history of the Universe. (See Big Bang)
o

BSL Version
oooo
oooo
oooo
oooo
oooo
What is other Elements?
o
- other elements formed;
– nucleus of the atoms of the light elements joined by nuclear fusion.
o
o - nuclear fusion of element atoms;
– happens deep inside the stars at the end of their lives.
o
o - lighter elements;
– such as oxygen and carbon ~ the first to form.
o
o - helium nuclei fuse;
– with oxygen and neon atoms to form atoms, such as silicon, magnesium and calcium.
o
o - heavy atoms form;
– when supergiant stars reach the end of their life and collapse ~ boosting the pressure of the gravity in their core. (see below)

oooo
oooo
oooo
oooo

BSL Version
oooo
oooo
Back to The Universe / next to Periodic Table page.